Programmed cell death (PCD) is an integral part of plant development. Still, it is largely unclear when developmentally controlled PCD (dPCD) has evolved and in how far the molecular control of different PCD pathways is conserved.
Different dPCD processes in the model plant Arabidopsis thaliana show a set of commonly upregulated "dPCD signature" genes (Olvera-Carrillo et al. 2015). These co-regulation in these genes independent of specific cell type that prepares PCD execution argues for the existence of a core dPCD pathway that gets activated regardless of the developmental context. Interestingly, many dPCD signature genes show orthologs within the land plant lineage (Olvera-Carrillo et al. 2015), suggesting that this hypothetical core dPCD pathway has evolved during terrestrialization - the colonization of land habitats by previously aquatic organisms - over 400 million years ago.
There are different approaches how to gain information on the evolution of dPCD in plants. One way is to investigate fossil records, which can give information on the morphology and histology of extinct plant species. Alternatively, comparative functional analyses of living species representing different branches of the plant tree of life can be undertaken, which allows to draw indirect conclusions about the presence or absence of molecular regulators in the hypothetical common ancestor of two given branches.
Following the latter approach, we investigated the molecular control of termination of flower receptivity by dPCD in the dicot Arabidopsis and in the monocot maize (Zea mays), representing two major plant lineages that have split over 100 million years ago. Arabidopsis and maize are not only evolutionary distant, but also exhibit drastically different flower morphologies and pollination strategies (see here). Nevertheless, we found that a close ortholog of the NAC transcription factor KIRA1 (KIR1), which controls stigma cell death and termination of receptivity in Arabidopsis (Gao, Daneva, et al. 2018), is responsible for the same function in maize: In maize, KIR1-like (KIL1) promotes senescence and degeneration of the style basis, effectively blocking the path for pollen tubes and therefore terminating flower receptivity (Šimášková, Daneva, et al. 2022).
These results suggest that KIR1 and KIL1 have either been used by the common ancestor of monocots and dicots to terminate floral receptivity by promoting dPCD, or that each ortholog has been recruited independently to perform this function. Given that a KIL1-ortholog also has been shown to promote pith dPCD in the hollow stems of the monocot Sorghum bicolor (Fujimoto et al. 2018), recruitment of KIL1-pathways to different developmental contexts is an intriguing possibility.
Encouraged by these results, we teamed up with Justin Goodrich (University of Edinburgh) and Gwyneth Ingram (ENS Lyon) to investigate the water-conducting tissue of Marchantia polymorpha, a representative of the basal land plant lineage liverworts. Marchantia does not produce a vascular tissue, and its flat bifurcating plant body ("thallus") does not produce roots, but is anchored in the substrate by tip-growing rhizoid cells that resemble root hairs. Some of these rhizoids have a smooth cell wall, and some produce finger-like cell wall protrusions into the cell lumen, and have been termed "pegged rhizoids". These pegged rhizoids also form bundles that run along the underside of the thallus in bundles reminiscent of vascular bundles. Therefore, pegged rhizoids have been implicated in water transport, in analogy to the xylem of vascular plants (Duckett et al. 2013).
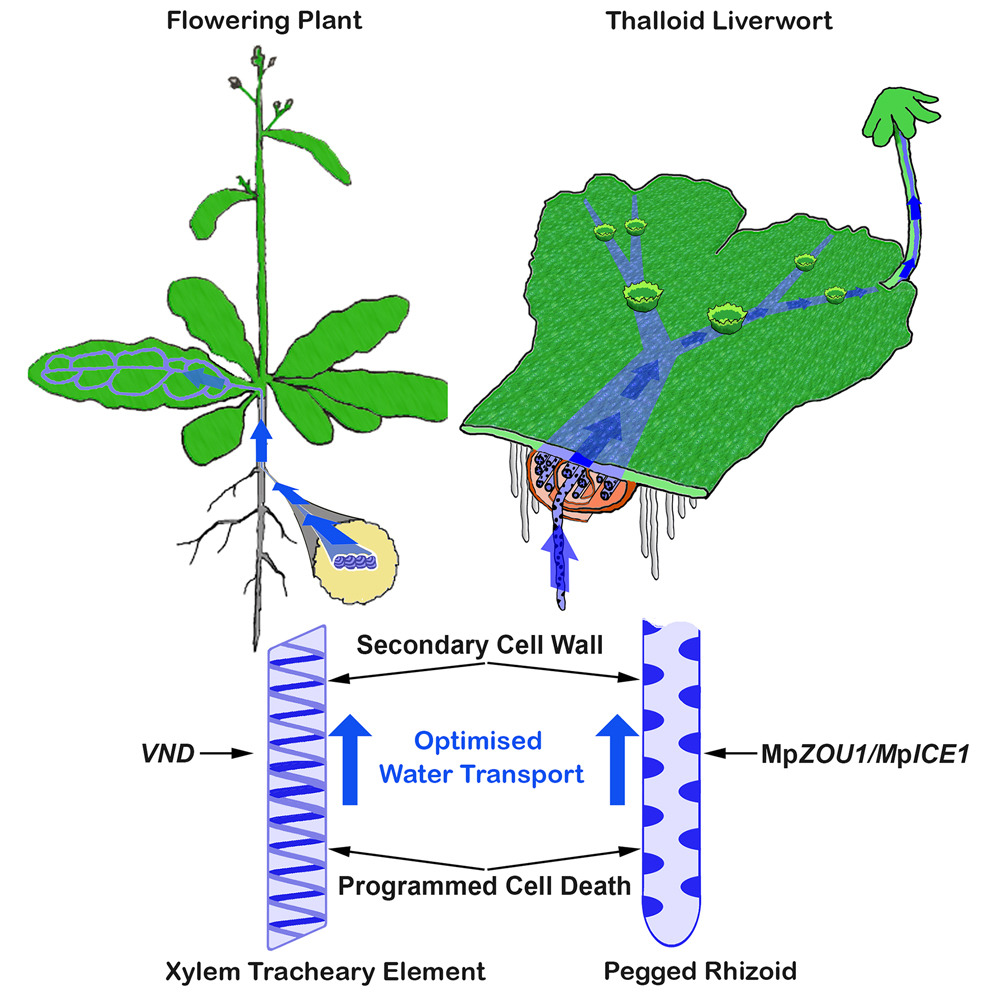
We identified a bHLH transcription factor, MpZOU1, which together with its interaction partner MpICE1 promotes pegged rhizoid formation in Marchantia (Lu et al. 2024). Like its functional but non-related vascular plan counterparts, the VND NAC transcription factors, MpZOU1 is important to control two central features of effective water conducting cells: Cell wall reinforcement and programmed cell death.